Immunotherapy Success Reinforces Immorta's Innovative Cancer Treatment Strategy

In today’s issue:
· Immorta Patents, Our Proprietary Stem Cell (pMSC) Triggers Organ Rejuvenation by Stimulation of Regulatory T Cells
· Cellular Rejuvenation, Higher Concentrations of Interleukin-35 Explain Potential Superiority of Immorta Bio’s Stem Cell Revivify™ Therapy in Liver Failure
· Senolytic Immunotherapy, Stunning Success of Immunotherapy Supports Immorta Approach to Treating Cancer
· Longevity Investments, A Preclinical Organ Regeneration Company Secures $65 Million in Series A Funding, Offering Strategic Insights for Immorta Bio's Fundraising Efforts
· Longevity Shorts, Immorta Bio Recruits Internationally Renowned Medical Luminary and Venture Capitalist to its Board of Directors
Immorta Patents
Immorta proprietary Stem Cell Triggers Regeneration by activation of Regulatory T Cells
On May 10th, 2024, Immorta Bio filed a provisional patent for the development of a novel category of stem cells, referred to as Personalized Mesenchymal Stem Cells (pMSC). This new cell type has demonstrated the ability to generate significantly more potent Regulatory T Cells compared to other MSCs, thereby exhibiting superior tissue and organ rejuvenation capabilities.
The patent application includes data demonstrating that Immorta’s pMSC product reduces liver failure by stimulating Regulatory T Cells. The ability of pMSC to generate these cells was found to be dependent on the cytokine interleukin-35 (IL-35). Notably, pMSC was shown to produce IL-35 at substantially higher levels compared to other mesenchymal stem cell types. The patent also encompasses various methods for enhancing IL-35 production from the Company’s pMSC, as well as numerous combinations with drugs, cells, and gene therapies.
Cellular Rejuvenation
A protein linked to extended survival inpatients with liver failure is produced at elevated levels by Immorta’s pMSCs
Scientists have discovered that patients with liver failure who have higher levels of the protein IL-35 experience significantly longer survival compared to those with lower levels. IL-35 plays a crucial role in the immune system by enhancing the production and activity of Regulatory T Cells, which suppress inflammation and promote organ regeneration. Data indicates that by increasing Regulatory T Cells activity, IL-35 can protect liver cells from damage and, in some cases, stimulate the production of new tissue.
Implications for Immorta
Immorta Bio’s cellular rejuvenation therapy is based on a proprietary process for generating personalized MSCs, known as pMSC. These pMSC produce significantly higher levels of IL-35 compared to MSCs produced through conventional methods and have shown superior efficacy in treating liver failure in animal models.
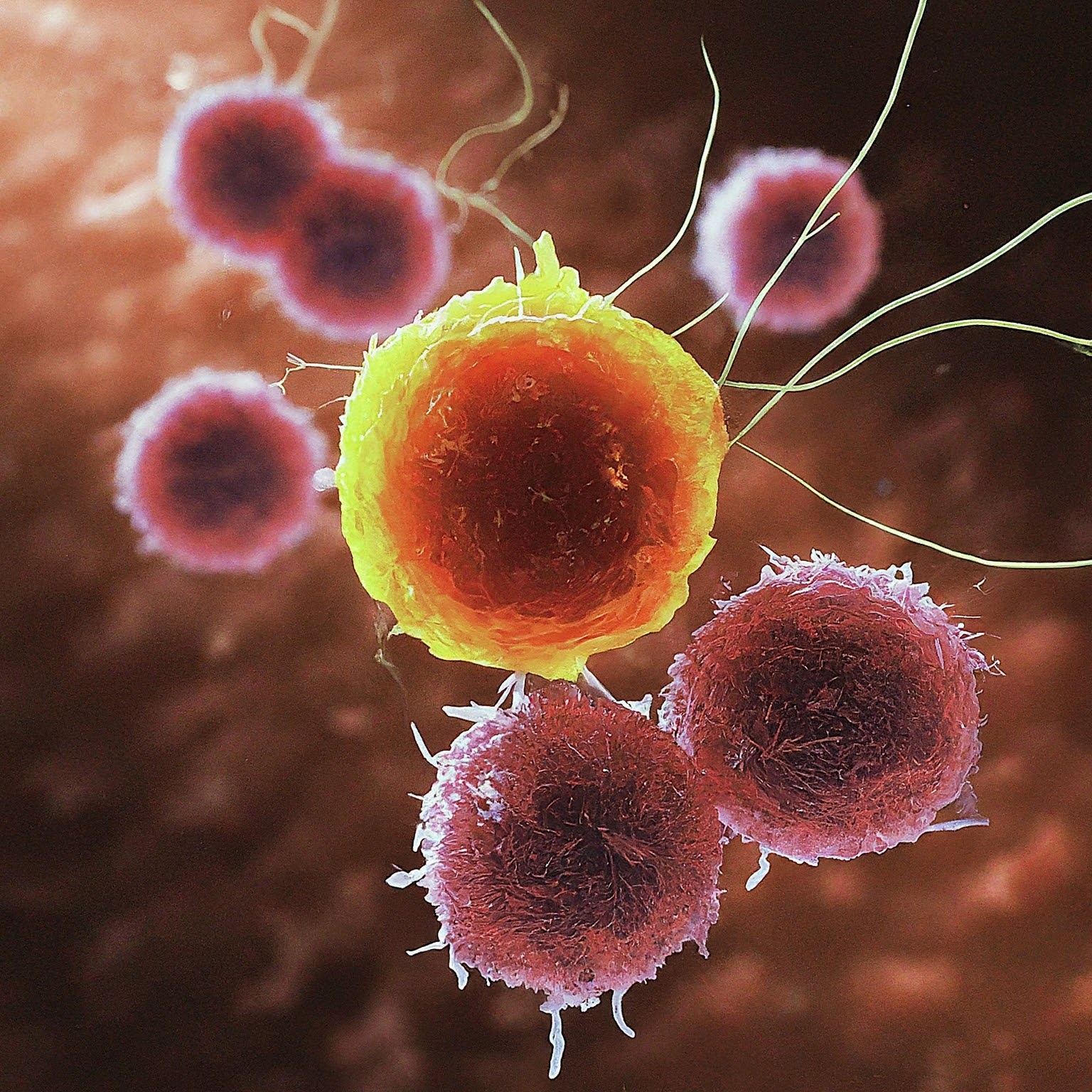
Senolytic Immunotherapy
Success in 12/12 cancer patients treated with immunotherapy provides hope to Immorta’s SenoVax™.
The immune system is designed to identify and eliminate cancer cells. Cancer cells often employ proteins known as “checkpoints” to protect themselves from immune attack. A prominent immunotherapy strategy involves the use of checkpoint inhibitors, effectively disrupting the mechanisms that suppress immune activity.
One molecule that acts as a checkpoint is PD-L1. In a recent clinical study involving a PD-L1 checkpoint inhibitor, all 12 patients exhibited a robust immune response resulting in the complete disappearance of their tumors, without the need for any additional therapies.
Implications for Immorta: SenoVax™ is an immunotherapeutic vaccine developed by Immorta Bio designed to activate the body's immune system to target and eliminate senescent cells that shield tumors from immune attack. It is believed that these senescent cells function as a checkpoint by suppressing the immune response against cancer cells.
Consistent with the published study, our preclinical experiments in animal models have shown complete tumore limination through the inhibition of senescent cells, without the need for additional therapies. This evidence supports the efficacy of checkpoint-focused immunotherapy as a standalone treatment, reinforcing the potential of our SenoVax™ approach.
Longevity Investments
Neural Regeneration Company Secures $65 Million to Advance Remyelination Drug to 'Proof of Concept' Clinical Trial
Progentos Therapeutics, a preclinical-stage company, has successfully completed a $65 million Series A funding round led by Forbion, a Dutch investment firm. The company is developing small-molecule therapeutics designed to activate progenitor cells in the brain to regenerate oligodendrocytes and replace myelin lost due to neurodegenerative diseases such as multiple sclerosis and age-related conditions. The funds will be used to advance the multiple sclerosis program to human proof-of-concept clinical trials and to expand the pipeline to address other neurodegenerative disorders.
Implications for Immorta
This capital raise underscores the strong investor interest in regenerative and longevity medicine and their willingness to invest substantial amounts in technologies at such an early stage of development.
Longevity Shorts
· Immorta Bio recruits internationally renowned medical luminary and Venture Capitalist, Dr. Russel Kaufman to its Board of Directors.
· Senescence is the culprit of COVID induced heart failure.
· Futurist, Ray Kurzweil, predicts AI will contribute significantly to radical life extension.
· David Sinclair’s patent on in vivo retro-differentiation by inducing expression of OCT4, NANOG and KLF4 has been published.
Boris Reznik, PhD
Chairman